
OneClass: Part C. Water of hydration analysis 1. Formula of anhydrous salt used マテ (formula of th...
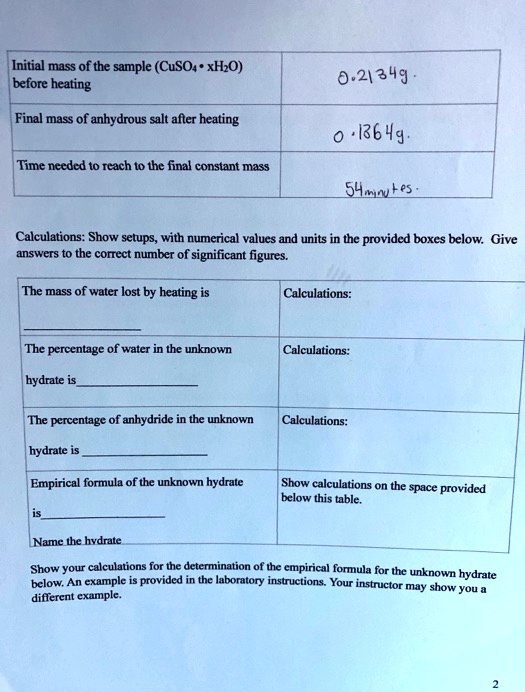
SOLVED: Initial mass of the sample (CuSO4 * xHO) before beating 0.21349 Final mass of anhydrous salt after heating Time needed t0 reach t0 the final constant mass S41uLes Calculations: Show setups;

5 g of crystalline salt, when rendered anhydrous, lost 1.89 g of water. The formula weight of anhydrous salts is 160 . The number of molecules of water of crystallisation in the salt is:

One mole of anhydrous salt AB dissolves in water and librates 21.0 J mol^-1 of heat. The value of ΔHydration AB is - 29.4 J mol^-1 . The heat of dissolution of
3 anhydrous salt to be 208.3 g. Using Equation 2, determine the number of moles of the anhydrous salt. Equation 2 Number of mol

thermodynamics - Why is it that the enthalpy of hydration equals the difference of solvation enthalpies of anhydrous and hydrous salts - Chemistry Stack Exchange

SOLVED: Formula (Molar) mass of anhydrous salt (on bottle) Number of grams of HzO per 100.0 g hydrate *49 14.8 Number of moles HzO per 100.0 g hydrate 1q.8 18.0 Da moles

One mole of anhydrous salt AB dissolves in water and librates 21.0 J mol ^-1 of heat. The value of Δ H(hydration) of AB is - 29.4 J mol ^-1 . The












.PNG)


